B04
Myeloid cells play an increasingly recognized role in CNS autoimmune conditions such as multiple sclerosis (MS). However, mechanisms that drive disease progression from the relapsing-remitting to the progressive stage and that cause accumulating neurologic damage are far from being definitive. We identified dynamic modes of communication between neurons, CNS-localized myeloid cells and invading T lymphocytes in chronic neuroinflammation. Now, we will link a detailed understanding of the molecular pathways underlying this metabolic communication with a strong translational focus on disease progression. We could demonstrate that enhanced metabolic activity e.g. tissues like tumors or regenerating tissues after injury or during inflammatory processes, promotes an M2-like macrophage phenotype that is orchestrated by the transcription factor ICER in a G protein-coupled receptor (GPCR)- and cAMP-dependent manner (Bohn et al., 2018). We propose that the inflammatory response in the CNS, and accompanying acidification of the tissue, instructs a M2-like tissue repair phenotype in macrophages counteracting neurodegenerative processes and disease progression as an intrinsic defence mechanism of the CNS. To this end, we intend to answer these questions in our working plan:
-
- What is the role of G protein-coupled sensing of acidification and ICER expression in CNS inflammation and disease progression?
- What role does the role of ICER in metabolic reprogramming of macrophages and microglia play in CNS inflammation?
- What is the role of ICER and GPCR for disease progression in MS patients?

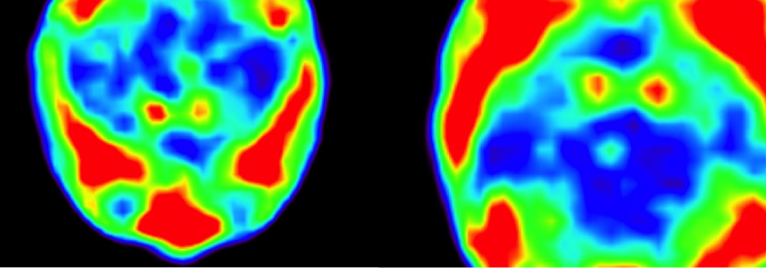
Sekine et al. Nature Immunology, 2018
Principal Investigators:
Prof. Dr. med. Stefan Bittner
Department of Neurology, Forschungszentrum Immuntherapie
Mainz
stefan.bittner@unimedizin-mainz.de
Univ.-Prof. Dr. rer. nat. Tobias Bopp
Institut für Immunologie Forschungszentrum Immuntherapie
Mainz
boppt@uni-mainz.de